While the article featured today is behind a paywall, here is the link to the paper anyway.
During my PhD, our department holds weekly seminars for graduate students to share their work with their peers and professors. Many of the graduate students within our department are associated with the Institute of Infectious Disease Research (IIDR) at McMaster University. Some of the IIDR labs focus on characterizing bacteria like P. aeruginosa. However, most of them are either developing new antibiotics, investigating bacterial responses to them, or optimizing their usage in the clinic. It’s because of IIDR that I have a deep interest in learning about fighting bacterial infections with antibiotics.
What is an antibiotic, though? An antibiotic is any kind of substance that acts against a microbe. Throughout history, humanity has used natural antibiotics like honey, plants, and even mould! Antibiotics are typically divided into two types: bactericidal and bacteriostatic. Bactericidal antibiotics destroy the bacterial cells, while bacteriostatic antibiotics prevent bacteria from growing further while keeping them intact. Both antibiotic classes target bacterial cell components essential for survival and growth. If you’ve ever had Strep throat, you may remember being prescribed Zithromax (azithromycin) or Cleocin (clindamycin), which are bactericidal and bacteriostatic antibiotics respectively.
Many of the antibiotics used to treat infections belong to the class of compounds called β-lactams (Figure 1). You may already be familiar with Penicillin, the first β-lactam to be discovered. In fact, penicillin was extracted from mould! β-lactams target a class of proteins called penicillin-binding proteins (PBP). These proteins are essential for bacterial cells to produce the cell wall which acts as a protective outer barrier (Figure 2). The red highlights in Figure 1 represent the core component of β-lactams: the β-lactam ring. The ability of β-lactams to destroy a critical component of a bacterial cell’s survival makes them bactericidal antibiotics.

Alexander Fleming, the scientist who discovered penicillin, warned about the emergence of penicillin resistance among the target bacteria in his 1945 Nobel Prize lecture 1. As per his warning, the prevalence of antibiotic-resistant infections is increasing worldwide2. The bacteria resistant to antibiotic treatment typically acquire or already have the means to counteract the antibiotic. Coming back to the β-lactams, bacteria can express proteins called β-lactamases that cut through the β-lactam ring. Cutting the β-lactam ring renders the β-lactams useless for treating infection. The increased prevalence of antibiotic resistance among infecting bacteria applies to every other class of antibiotic that you can think of. The rise of antibiotic-resistant bacteria, in turn, is projected to kill almost 400,000 Canadians and cost $120 billion in hospital expenses over the next three decades3.
With the alarm bells of antibiotic resistance ringing, research labs like IIDR are now hard at work to develop new antibiotics to treat infectious diseases. Today, however, I will talk about a paper I came across on Facebook about a potentially novel class of antibiotics4. In this paper, researchers from multiple pharmaceutical companies such as Entasis and Novartis tweaked the antibiotic avibactam (Figure 3, left), which contains a diazabicyclooctane (DBO). DBOs are a bicyclical compound that looks like a heptagon if you drew it in 2D space (see the 7-sided drawing with the nitrogen (N) atoms in Figure 3, left). Avibactam is intended to be a β-lactamase inhibitor that does not bind to PBPs as β-lactams do. Nevertheless, the authors sought to change that by increasing the compound’s binding to the PBPs essential for bacterial growth. To that end, the authors began by modifying avibactam’s structure by fusing a pyrazole ring (Figure 3, right) to the 3rd and 4th member of the 7-membered ring in avibactam (see blue numbers in Figure 3, left).
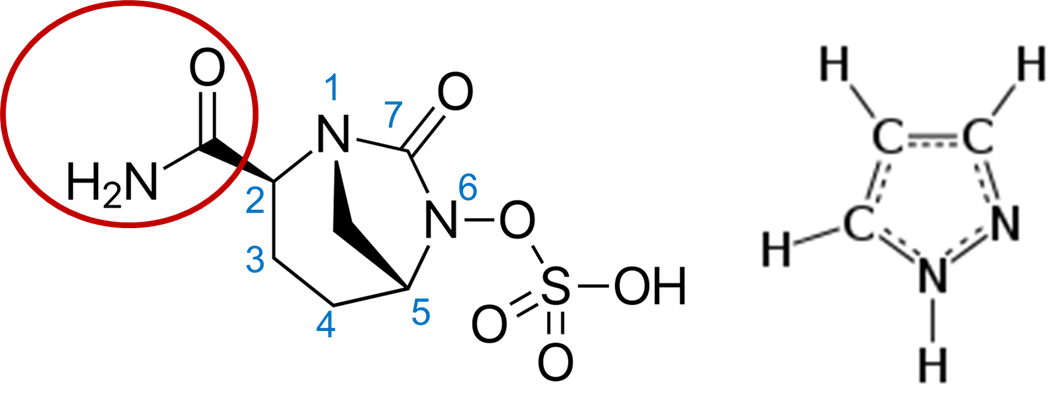
Many compounds can have functional side groups (eg. the red circle in the left image of Figure 3), a group of atoms having distinctive chemical properties when bound together. Modifications to these side groups can change a compound’s function in the environment. The authors modified the side groups in their fused compound to overcome two problems. The first set of modifications further increased the compound’s binding against PBP1 and PBP3 instead of PBP2. You see, there are also multiple types of PBPs. PBP2 is not as essential for bacterial growth, but PBP1 and PBP3, which both S. maltophilia and P. aeruginosa express, are essential. This increased binding, in turn, makes the cells more sensitive to killing while being resistant to degradation by β-lactamases!
The second set of modifications was done so that the antibiotic can enter the cell. P. aeruginosa has a high intrinsic antibiotic resistance. This means that the cell can handle high concentrations of antibiotics before they start dying even without any genetic modifications. P. aeruginosa has a tight cell wall that makes it difficult for antibiotics to enter the cell. Even after the initial side chain modifications, some P. aeruginosa strains were still resistant to the fused compound, attributed to poor antibiotic entry. Nevertheless, P. aeruginosa can express porins, barrel proteins acting as a pore for nutrients to enter the cell. The authors surmised then that modifying other side chains in the fused compound would enable the compound to enter P. aeruginosa more efficiently using porins. The results from the study suggested that modifying these side groups further increased entry into the cells that used to be resistant to the fused compound!
Now the authors had a compound that could enter the cell and prevent cell wall synthesis through PBP1 and PBP3 binding. This compound was named ETX0462. But could the compound treat microbial infections? In a final series of experiments, the authors treated mice infected by B. pseudomallei, another pathogen, with EXT0462 to see how long they would survive. Almost half of the mice treated with ETX0462 two hours after initial infection survived after 25 days, with every mouse surviving if ETX0462 was administered a day after initial infection. In contrast, all the mice treated with ceftazidime, an antibiotic already on the market, died less than 5 days after infection. This was also the case with the control untreated group. When examining viable bacterial counts in lung and thigh infections treated with ETX0462, concentrations as low as 50 mg/kg (typical dose for mouse infection) every 3 hours reduced bacterial counts by at least 99.99% in both cases. I considered both sets of data to be quite promising for ETX0462 as a novel antibiotic!
With all the data so neatly presented, I became excited at the prospect of ETX0462 hitting the market one day. Nonetheless, questions remain for ETX0462. For one, ETX0462 would still require further testing through clinical trials to ensure the compound’s safety for patients infected by P. aeruginosa and S. maltophilia. The full range of the compound’s activity against other clinical strains of the bacterium also remains to be seen. That being despite showing ETX0462’s efficacy against other Gram-Negative pathogens like K. pneumoniae and E. coli. Finally, the study does not address how quickly the targeted bacteria would accumulate resistance against ETX0462 if the antibiotic was regularly used. A series of mutations that alter the antibiotic’s binding site in PBP3 could very easily evade antibiotic treatment as the authors showed. Furthermore, the compound could be shuttled out of the cell with efflux pumps, as is the case for many other classes of antibiotics targeting P. aeruginosa.
Antibiotic resistance remains a major challenge for researchers and clinicians. However, ETX0462 seems to be a promising start for treating a range of bacterial species commonly observed in human infections. What I loved most about this paper was the step-by-step process the authors undertook to modify avibactam to grant the antibiotic additional power. Starting as just a β-lactamase inhibitor, ETX0462 now can inhibit essential PBPs too! Much work remains to be done for ETX0462 and other novel antibiotics, but being able to refine these antibiotics further for clinical use provides an important step to making sure antibiotic treatment remains effective.
References
- https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf
- Nathan, C., and Cars, O. (2014). Antibiotic resistance–problems, progress, and prospects. N. Engl. J. Med. 371, 1761–1763.
- https://www.cbc.ca/news/health/superbug-deaths-1.4429406
- Durand-Reville, T.F., Miller, A.A., O’Donnell, J.P., Wu, X., Sylvester, M.A., Guler, S., Iyer, R., Shapiro, A.B., Carter, N.M., Velez-Vega, C., et al. (2021). Rational design of a new antibiotic class for drug-resistant infections. Nature 597, 698–702.
