Microbial cultures and human cultures are not the same
When you think of the word “culture”, what’s the first word that comes to mind? We can discuss a workplace culture where employees and employers share common attitudes and values about a company’s work and vision. We can also talk about the culture of a nation or ethnicity based on their shared artistic works and achievements. These definitions of culture focus on identifying common traits between groups of people. In science, microbiologists use the word “culture” when they grow and maintain microbes in the lab. Under this definition, we have traits shared between the microbes that can grow in a specific “culture” condition.
Bacterial cultures: helping bacteria grow since 1860
The practice of isolating microbes using cultures began in the 19th century. in 1860, Louis Pasteur created what he called “yeast soup” for isolating bacteria that could digest specific sets of molecules to grow1. Today, microbiologists can grow bacteria in liquid media by forming a solution comprised of different nutrients. This solution provides the conditions necessary for microbes to grow. When microbes grow in liquid media, the solution becomes cloudy or hazy (Figure 1). The billions of cells suspended in the media create this cloudy appearance. Once fully grown, microbiologists can isolate and study these bacteria further. Alternatively, they can also store bacteria using a storage solution (typically 50% glycerol) and cryopreserving them (freezing bacteria at extremely low temperatures).

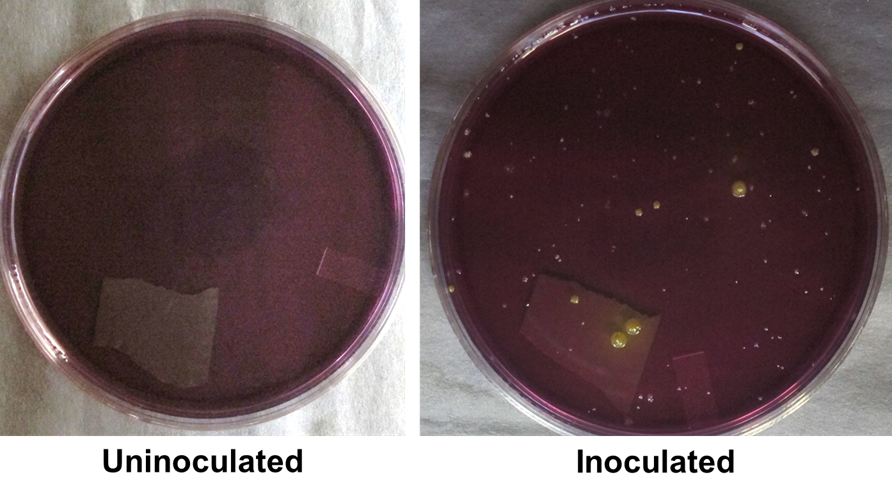
Soon after Louis Pasteur made his yeast soup, another scientist named Julius Richard Petri developed a method for cultivating microbes using a circular dish. He named his invention the Petri dish (used in Figure 2). The Petri dish is a shallow, circular dish that is filled with a substance called agar. If you’ve ever made agar jam before, the agar used to make jam is the same kind of material used to make bacterial plates. The agar used in bacterial cultures begins as a powder. This powder is mixed with a solution filled with the nutrients needed for microbes to grow. Once heated and cooled, this suspension solidifies into an agar jelly (Figure 2, Left).
This jelly now acts as a platform for inoculating microbes from all kinds of places. This can be a nasal swab, a freshwater sample, or even your fingers! After spreading a sample across a plate, colonies will appear when the plate is incubated for some time (Figure 2; Right). These colonies comprise individual microbial cells clumped together and can come in many shapes and sizes. Even within the same species, colonies can look very different on the same plate.
E. coli the microbial bodybuilder: feasting on protein supplements and sugars
Since the 19th century, microbiologists have come up with other ways to isolate microbes. In the 1950s, microbiologists were trying to grow a universal microbe called Escherichia coli (or E. coli). These efforts led to making a rich medium called Lysogeny Broth, or LB. LB has 10 grams of proteins derived from cow’s milk and 5 grams of yeast extract per Litre. This amount of protein and sugar would be enough to make a bodybuilder out of microbes like E. coli. Even though we can’t see an E. coli cell with our naked eyes, E. coli populations can grow to over 10 million cells in just a few hours after being introduced to the medium.
We have a long way to go to culturing the buggers
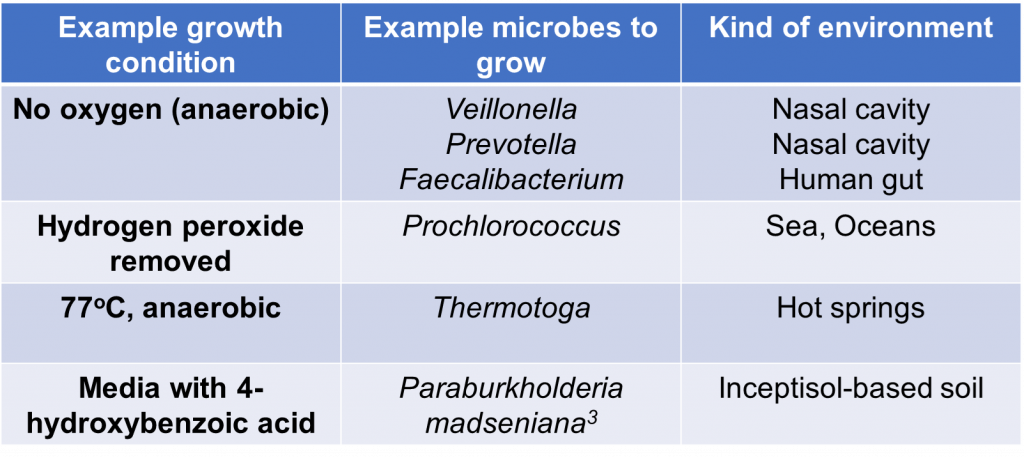
The real challenge of growing microbes, however, comes from the challenges in documenting every microbial species that grow in our world. As of today, the scientific community has only cultured an extremely small minority of the world’s microbial species. With rich media like LB being widely available in the lab, why is culturing microbes still so difficult? To better understand this conundrum, let’s compare ourselves with animals for a moment. Humans can eat all kinds of food that other animals can’t. On the one hand, I love chowing down on chocolate, but dogs cannot eat chocolate at all. On the other hand, cows can eat grass while humans can’t. In the same way, many bacteria will not grow even with all the nutrients provided in LB because they don’t want or like it. For example, two species of oral-associated bacteria – Prevotella sp. HOT-376 and Fretibacterium fastidosum – required specific molecules called siderophores in the media to grow2. Since these molecules are not present in LB, the two species will not grow in LB. There are many more examples of microbes being very picky with their ideal home for growth, some of which I list in Table 1.
Now let me extend this problem for microbes growing outside our bodies. First, the microbial world is vast. One paper used ecological scaling laws to show that there could be as many as 1 trillion microbial species on Earth4! That’s almost 1000 times the number of humans living on Earth! Furthermore, the total number of bacterial cells on Earth outnumbers the number of stars in the universe (5 million trillion trillion bacterial cells5 vs 200 sextillion stars in the universe6)! Most of the unknown microbial species live in seawater, freshwater, soil, and other terrestrial environments as extreme as hot springs and hydrothermal vents7. Microbiologists also know that these bacteria are extremely picky with the nutrients they need to grow. They also don’t know what nutrients the microbes want, compounding the problems further. It’s like having a new pet and not knowing what they can and can’t eat. The sheer number of microbial species and their picky tendencies should highlight how difficult profiling all the microbes in the world can truly get.
Culture-enrichment: promise in surveying the microbial world
With culturing environmental microbes being so difficult, how can microbiologists come close to characterizing the microbes that live in our world? The answer comes in the form of culture-enriched profiling. This approach focuses on using multiple kinds of culture plates and growth conditions to isolate as many kinds of bacteria as possible. If you check out Table 1, you can see some examples of growth conditions that microbes from diverse environments need to grow.
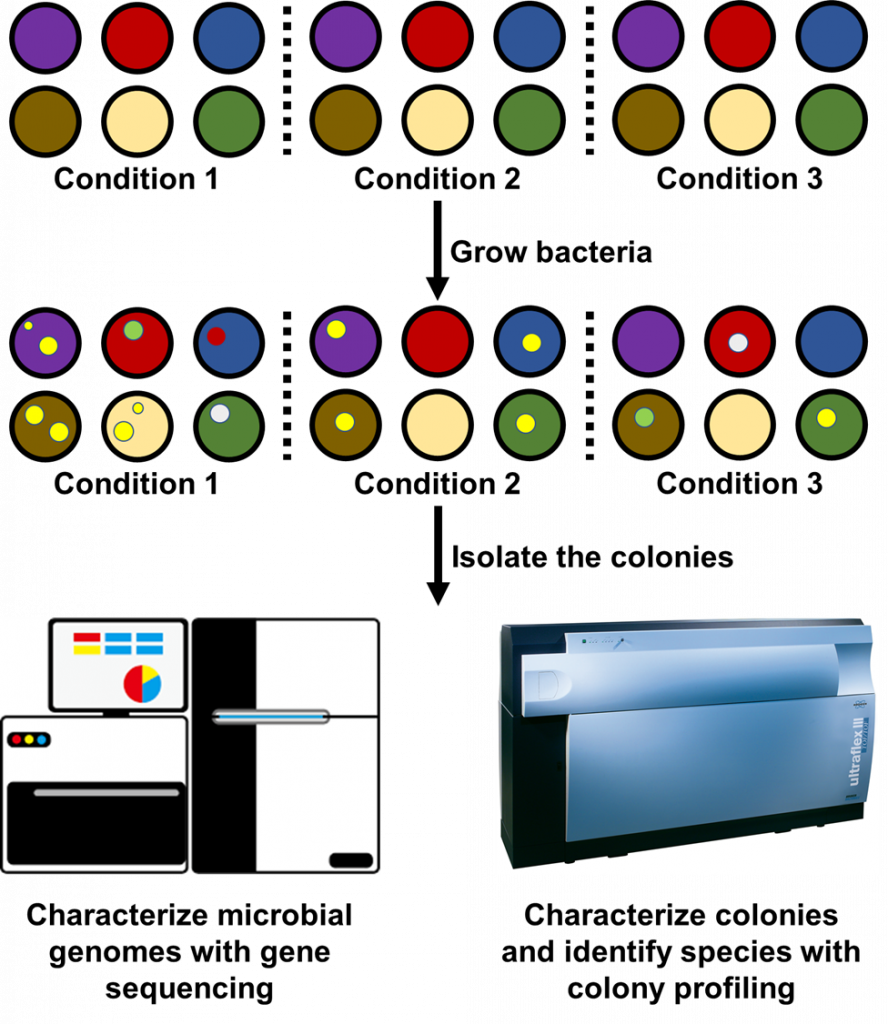
Because microbiologists don’t know when they’ve captured the entire microbial community, they may employ hundreds of culture plates and growth conditions at once to identify new species (Figure 3). Microbiologists have used it to identify 31 new bacterial species in the human gut8. My lab has also used a similar approach to obtain a complete profile of the microbial communities infecting the airways of cystic fibrosis patients9. Just the past year another research group developed a protocol for isolating hundreds of bacterial species from plant roots10. With the growing literature on cultivating microbes, microbiologists can now focus on brainstorming conditions that would encourage the growth of new bacteria. The ability to prepare all kinds of culture plates is also steering microbiologists ever closer to profiling entire microbial communities, at least for those in the body.
It’s amazing that over 100 years ago we were only beginning to isolate the seemingly infinite numbers of bacteria teeming underneath our eyes. We have come so far to studying the complete sets of microbes that grow in our bodies by culturing them. Extensive culture-enriched profiling methods show that at the very least, substantial portions of the microbial world can be isolated in the lab when the right lab conditions are provided. Even so, microbiologists still hold the belief that they cannot isolate the vast majority of bacteria in the environment. While this attitude may hold true for some environments, it is only a matter of time before microbiologists begin uncovering all the microbes that grow in our world.
References
- Bonnet, M., Lagier, J.C., Raoult, D., and Khelaifia, S. (2020). Bacterial culture through selective and non-selective conditions: the evolution of culture media in clinical microbiology. New Microbes New Infect. 34, 100622.
- Vartoukian, S.R., Adamowska, A., Lawlor, M., Moazzez, R., Dewhirst, F.E., and Wade, W.G. (2016). In Vitro Cultivation of “Unculturable” Oral Bacteria, Facilitated by Community Culture and Media Supplementation with Siderophores. PLoS One 11, e0146926.
- Wilhelm, R.C., Murphy, S.J.L., Feriancek, N.M., Karasz, D.C., DeRito, C.M., Newman, J.D., and Buckley, D.H. (2020). Paraburkholderia madseniana sp. nov., a phenolic acid-degrading bacterium isolated from acidic forest soil. Int. J. Syst. Evol. Microbiol. 70, 2137–2146.
- Locey, K.J., and Lennon, J.T. (2016). Scaling laws predict global microbial diversity. Proc. Natl. Acad. Sci. U S A 113, 5970–5975.
- (2011). Microbiology by numbers. Nat. Rev. Microbiol. 9, 628–628.
- Jackson, B. September 20th, 2021. The Conversation. https://theconversation.com/how-many-stars-are-there-in-space-165370
- Lloyd, K.G., Steen, A.D., Ladau, J., Yin, J., and Crosby, L. (2018). Phylogenetically Novel Uncultured Microbial Cells Dominate Earth Microbiomes. mSystems 3, e00055-18.
- Lagier, J.-C., Dubourg, G., Million, M., Cadoret, F., Bilen, M., Fenollar, F., Levasseur, A., Rolain, J.-M., Fournier, P.-E., and Raoult, D. (2018). Culturing the human microbiota and culturomics. Nat. Rev. Microbiol. 16, 540–550.
- Whelan, F.J., Waddell, B., Syed, S.A., Shekarriz, S., Rabin, H.R., Parkins, M.D., and Surette, M.G. (2020). Culture-enriched metagenomic sequencing enables in-depth profiling of the cystic fibrosis lung microbiota. Nat. Microbiol. 5, 379–390.
- Zhang, J., Liu, Y.-X., Guo, X., Qin, Y., Garrido-Oter, R., Schulze-Lefert, P., and Bai, Y. (2021). High-throughput cultivation and identification of bacteria from the plant root microbiota. Nat. Protoc. 16, 988–1012.
